Silver Ion-exchanged Glasses
Commercial glasses are silver exchanged and locally treated with UV light to form controlled distributions of silver nanoparticles or nanoholes on the film surface, for sensor applications.
Growth of silver nanoparticles by simultaneous continuous ultraviolet irradiation and heat treatment(1)
Silver-exchanged soda-lime glasses have been obtained by immersing 3-mm-thick commercial soda-lime glasses for 20 min at 320°C into a molten salt bath of molar concentration 0.1% AgNO3 in NaNO3. M-lines technique has been performed in order to determine the refractive index of the exchanged-glasses which varies from 1.61 to 1.52 across a depth of 8 µm.
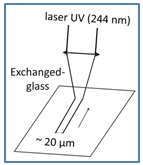
We have performed ultraviolet irradiation with a 244 nm continuous wave laser, which has been focused on the surface of the exchanged-glass samples. The samples have been placed on a hot plate to perform thermal annealing between room temperature (RT) and 450°C. Lines of 20 µm to 3 mm-width have been written, depending on the focus of the laser.
Absorption spectra have been recorded using a confocal spectrometer used in the transmission mode and equipped with halogen and deuterium lamps.
The absorption band centered at 350 nm is attributed to silver atoms (2) and the 235 nm-centered one to silver ions (3).
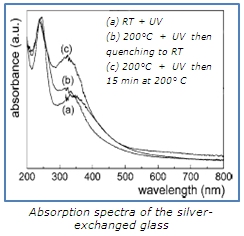 The interpretation of these spectra leads to the conclusion that simultaneous treatments (UV and heat) favor the reduction and limit the oxidation to the benefit of precipitation: the UV exposure allows reducing silver ions into atoms and the simultaneous heating enhances the reduction efficiency (1).
The interpretation of these spectra leads to the conclusion that simultaneous treatments (UV and heat) favor the reduction and limit the oxidation to the benefit of precipitation: the UV exposure allows reducing silver ions into atoms and the simultaneous heating enhances the reduction efficiency (1).
Absorption spectra have been recorded using a confocal spectrometer used in the transmission mode and equipped with halogen and deuterium lamps.
The absorption band centered at 350 nm is attributed to silver atoms (2) and the 235 nm-centered one to silver ions (3).
 The interpretation of these spectra leads to the conclusion that simultaneous treatments (UV and heat) favor the reduction and limit the oxidation to the benefit of precipitation: the UV exposure allows reducing silver ions into atoms and the simultaneous heating enhances the reduction efficiency (1).
The interpretation of these spectra leads to the conclusion that simultaneous treatments (UV and heat) favor the reduction and limit the oxidation to the benefit of precipitation: the UV exposure allows reducing silver ions into atoms and the simultaneous heating enhances the reduction efficiency (1).
With annealing temperature of 450°C, an unusual color (intense red) of the irradiated area appears. It highlights the presence of large NPs whose diameter depends on the UV dose and the annealing temperature. To explain this formation, we propose that small silver NPs of diameters of few nanometers are formed almost immediately under high temperature UV exposure. These NPs are further UV exposed due to the low laser scanning speed compared to the NPs’ growth kinetic. Thus, larger NPs are formed, mainly located near the glass surface and, in the same time, some earlier formed NPs are dissolved rendering available some silver atoms to coalesce into larger NPs.
As a conclusion, our experimental technique enhances the glass photosensitivity compared to similar treatments performed in two separated steps, by avoiding oxidation of silver atoms at high temperature to the benefit of the NPs precipitation.
(1) For more information, see: F. Goutaland, E. Marin, J. Y. Michalon and A. Boukenter, Applied Physics Letters 94, 181108 (2009).
(2) F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles, Wiley, New York (1998).
(3) J. Qiu, M. Shirai, T. Nakaya, J. Si, X. Jiang, C. Zhu, and K. Hirao, Applied Physics Letters 81, 3040 (2002).
Growth of silver nanoparticles by continuous ultraviolet irradiation
 The experimental technique explained above permits to obtain NPs whose diameters don’t exceed few nanometers. The aim of this study is to grown silver NPs with diameter up to 10 nm.
The experimental technique explained above permits to obtain NPs whose diameters don’t exceed few nanometers. The aim of this study is to grown silver NPs with diameter up to 10 nm.
During UV irradiation, highly concentrated silver atoms are formed through laser-induced reduction. This high concentration shortens the diffusion length, rendering sufficient enough the mobility of silver atoms at RT for precipitation. Thus, thanks to the high photosensitivity of the exchanged-glasses, the UV irradiation with power density correctly adjusted is sufficient to grow large NPs and no thermal annealing is required.
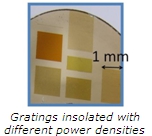 We have written different gratings of 3 mm-length and 30 µm-period with different power densities ranging from 25 kW/cm2 to 35 kW/cm2. The coloration of the insolated area varies with the power density (see figure on the left).
We have written different gratings of 3 mm-length and 30 µm-period with different power densities ranging from 25 kW/cm2 to 35 kW/cm2. The coloration of the insolated area varies with the power density (see figure on the left).
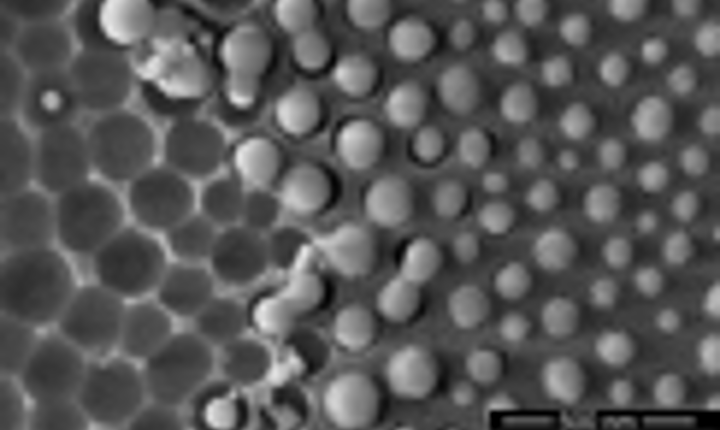
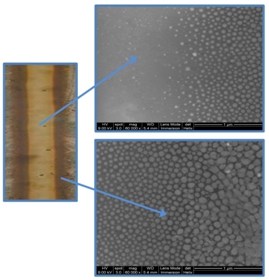 Small NPs are located on the insolated line (not visible in SEM view but revealed by Plasmon resonance in absorption spectra). Of both sides of the laser line, larger NPs are spread on a width of 2-3 µm. The sizes of these NPs increases when looking further away from the edge of the laser-written line.
Small NPs are located on the insolated line (not visible in SEM view but revealed by Plasmon resonance in absorption spectra). Of both sides of the laser line, larger NPs are spread on a width of 2-3 µm. The sizes of these NPs increases when looking further away from the edge of the laser-written line.
We focus our researches now on two main applications: detection of various chemical species through Surface Enhanced Raman Scattering effect for the glass containing large NPs.
formation of nanovoids of about 40 nm in diameter after careful cleaning of the UV-exposed glass surface.
The samples are studied by optical microscopy, by scanning electron microscopy (SEM) and by absorption spectroscopy.