Article: Alternative local melting-solidification of suspended nanoparticles for heterostructure formation enabled by pulsed laser irradiation
Last October, an article co-authored by Tatiana Itina, a member of our Laser Matter Interaction team, was featured on the cover of Advanced Functional Materials, a journal publishing outstanding research on improving the chemical and physical properties of materials.
In their article, the team puts forward a mechanism for nanoparticle phase change, sintering and growth in pulsed laser irradiation of suspensions (PLIS). Using atomistic simulations such as reactive bond molecular dynamics and density functional theory, as well as experimental observations, PLIS is shown to be promising for the synthesis of submicron heterostructures. The synthesized submicron Cu-CuO-Cu2O heterostructures containing Cu2O3 phase are thoroughly investigated for application in ethanol oxidation fuel cells. The article is the outcome of a follow-up study conducted by the Franco-Polish team, building upon their previous work in the now-completed project carried out within the framework of the PHC POLONIUM program.
Figure 1 below: Timeline of new species formation on the decomposition curves of ethanol for the Cu-ethanol and CuO-ethanol systems obtained from the simulation, where (I) ethanol absorption, (II) hydroxyl cleavage, (III) formation of Cu clusters, (IV) surface oxidation, (V) further decomposition of the solvent, and (VI) net oxidation
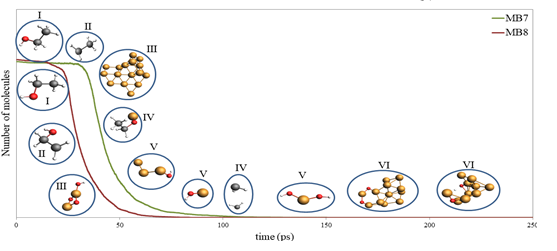
Figure 2 below:
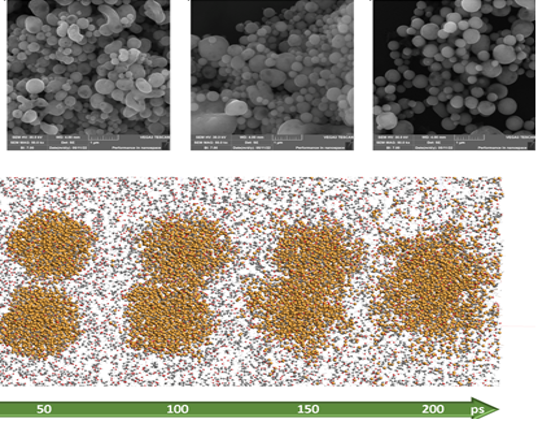
EM images of CuO-ethanol samples (upper road) and reactive MD simulation results (lower road) showing. The increasing trend of particle size with laser fluence is clearly visible.
Abstract
Phase formation by pulsed laser irradiation of suspended nanoparticles has recently been introduced as a promising synthesis technique for heterostructures. The main challenge still lingers regarding the exact mechanism of particle formation due to the non-equilibrium kinetic by-products resulting from the localized alternative, fast, high-temperature nature of the process. Here, we analyze the bond breaking/formation of copper or copper (II) interfaces with ethanol during the absorption of pulses for Cu-CuO-Cu2O formation applicable as a hyper-electrocatalyst in ethanol oxidation fuel cells. This study includes but is not limited to, a comprehensive discussion of laser-suspension interaction for practical control of the synthesis process. We have shown that beyond the physical transitions, the local interface between dissociated ethanol and the molten sphere is responsible for the oxidative/reductive interactions resulting in the formation of catalytic-augmented Cu3+ by-product, thanks to the reactive bond force field molecular dynamics studies confirmed by ab-initio calculations and experimental observations.
Read the full article here